| |
 |
Abstract |
|
| |
| Vol 46 No. 8: 691-698 |
[PDF] [Full Text] |
| |
| Selection of HBV preS1-binding penta-peptides by phage display |
| |
| Yonggang He1,†,
Xiaoli Ye1,†,
Pierre Tiollais2,
Jiming Zhang3,
Junqi Zhang1,
Jing Liu1,* and
Youhua Xie1,*
|
1Key Laboratory of Medical Molecular Virology (Ministry of Health and Ministry of Education), Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, Shanghai 200031, China
2Unité d'Organisation Nucléaire et Oncogénèse, INSERM U993/Institut Pasteur, 75724 Paris, France
3Department of Infectious Disease, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai 200040, China
† These authors contributed equally to this work. |
| |
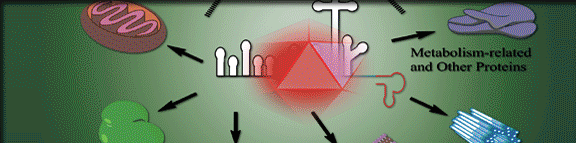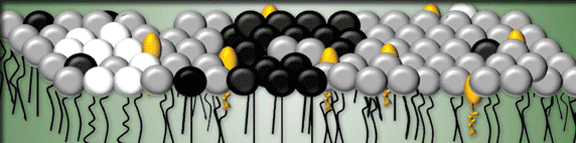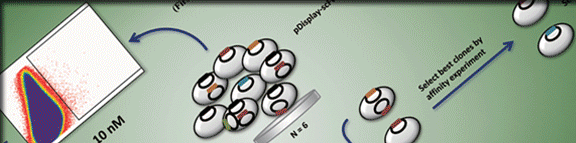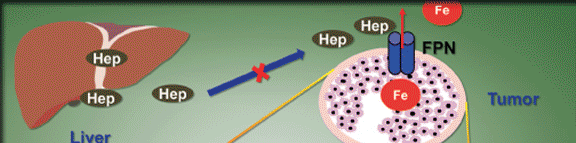
Abstract Chronic hepatitis B virus (HBV) infection can lead to liver cirrhosis and hepatocellular carcinoma. Current therapies have a very limited efficacy in virus clearance. New antiviral targets and agents are urgently needed. The envelope of HBV virion contains three surface glycoproteins, namely the large (LHBs), middle (MHBs), and small (SHBs) proteins. LHBs has an amino terminal preS which is composed of the preS1 and preS2 domains. The amino half of preS1 which is myristoylated plays a pivotal role in HBV entry, which can be exploited as an antiviral target. A common motif of five amino acids had been previously discovered to bind preS11–65 and HBV particles. In this study, we used preS11–65 to screen a phage display library of random penta-peptides to select the penta-peptides possessing a high preS1-binding affinity. After nine rounds of panning, we obtained one peptide designated as A5 which could bind preS1 with a high affinity. By systematically substituting each residue of A5 with the other 19 amino acids, we identified a novel peptide with an increased preS1-binding affinity. Both peptides could inhibit HBV attachment to HepG2 cells, making them be potential candidates for HBV entry inhibitors. |
| |
Keywords HBV; preS1; PIII; phage display; peptide |
| |
Received 2014-1-29
Accepted 2014-3-7 |
| |
Funding This work was supported by the grants from the National Key Project for Infectious Diseases of China (2008ZX10002-010, 2012ZX10002-006, 2012ZX10004-503, and 2013ZX10002-001), the National Basic Research Program of China (2012CB519002), the Natural Science |
| |
* Correspondence address Tel: +86-21-54237972; Fax: +86-21-54237973; E-mail: [email protected] (Y.X.)/[email protected] (J.L.) |
| |
| Browse:1044 |
|
 |
|
|
| Manuscript Submission |
|
|
 |
|
|