|
http://www.abbs.info e-mail:[email protected] ISSN 0582-9879 ACTA BIOCHIMICA et BIOPHYSICA SINICA 2002, 34(2): 138-142 CN 31-1300/Q |
Mouse
Restin Inhibits Bovine Aortic Endothelial Cell Proliferation and Causes Cell
Apoptosis
(
Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, the Chinese Academy of Science,
Shanghai
200031, China; 1Shanghai University of Traditional Chinese
Medicine, Shanghai 200032, China )
A
significantly homologous protein found by Ramchandran et al was the
C-terminus 184 amino acids of mouse collagen XV alpha 1 chain. Type XV collagen is composed of 1 363
amino acids. It contains a highly
interrupted collagenous (COL) region of 577 residues, and noncollagenous (NC) amino- and carboxyl-terminal domain
of 530 and 256 residues[5].
Type XV collagen presented in many tissues as evidenced by strong
association with vascular,
neural, mesenchymal and
some epithelial basement membrane zones[6]. Ramchandran et al[7]
found that the recombinant C-terminus of human collagen XV (restin) could
inhibit the migration of endothelial cells in vitro but had no effect on
the proliferation of these cells.
In our experiments, the
result showed that recombinant mouse restin could inhibit the proliferation of
endothelial cells in vivo.
Treatment of BAE cells with recombinant restin caused G0/G1
arrest and cell apoptosis.
The
cDNA of C-terminus of collagen XV alpha 1 chain was amplified from total RNA of
mouse muscle and was cloned into the expression plasmid pQE32. After induction with IPTG, the recombinant protein was expressed
in inclusion body and accounted for 60%-70%
of the total E.coli protein.
The recombinant protein was purified and refolded. It inhibited BAE cell proliferation
stimulated by bFGF as a dose-dependent manner. Flow cytometry and annexin V-FITC binding assay demonstrated
that treatment of BAE cell with recombinant protein caused the changes of cell
cycles and cell apoptosis.
1.1
Reagents and materials
DMEM
and Trypsin/EDTA were purchased from Gibco BRL(Rockville, MD). Fetal calf serum was from Hyclone (Logen UH). Mouse was from Shanghai Animal Center. Endostatin was prepared in our
lab. BAE cells were isolated as
previously reported from bovine aortic[8].
1.2
RT-PCR
Mouse
fresh muscle was homogenized in liquid nitrogen. Total RNA was isolated using
Trizol reagent (Gibco BRL). Total
RNA was used as the template for cDNA synthesis using SuperscriptTM
RNase H- transcriptase (Gibco BRL) according to manufacturer's
instructions. PCR was performed with Ex-Taq DNA polymerase (TaKaRa)
according to manufacturer's instructions. The synthetic oligo-nucleotides were
obtained from Shanghai Sangon Co. Ltd. (Shanghai, China). The primers used were
as follows: RT primer: 5′-TTATTACTTCCTAG-TGTCTGTCATGAAAC-3′,
sense primer: 5′-ATT-TTAAGTGCCAACTATGAGAGGCCT-3′,antisense
primer: 5′-TTATTACTTCCTAGTGTCTGTCATG-AAAC-3′,
two stop codons were included in the antisense primer. PCR product of 560 bp was amplified
with this primer sets. Reaction
was incubated in PE480 thermal cycler (Perkin-Elmers, NJ) for 35 cycles:
denaturation 30 s, 94 ℃; annealing 30 s, 55 ℃; extension 30 s, 72 ℃. PCR product was run on 1% agarose gel
in TBE (10 mmol/L Tris-borate, 1
mmol/L EDTA, pH 8.0), and visualized by ethidium bromide
staining.
1.3
Plasmid construction
The
amplified cDNA fragment was ligated into Escherichia coli
expression vector pQE32 (Qiagen),
resulting in the construction of an expression plasmid pQEXV. The restin containing vector was
sequenced.
1.4
Purification and refolding of recombinant restin
pQEXV
was transformed into E.coli M15 (Qiagen) and mouse restin expression was
induced by 1 mmol/L IPTG. Cells
were harvested by centrifugation for 10 min at 4 000 g. Cells were resuspended in 20 mmol/L
Tris-HCl, pH 8.0, 50 mmol/L KCl, 0.5 mmol/L EDTA, 5 mmol/L DTT and lysozyme was added to
the final concentration of 0.5 g/L.
Cells were incubated at 4 ℃
for 30 min, then were disrupted by
sonic homogenizer for 10 s for ten times with 30 s interval each time. After centrifugation at 4 ℃,
12 000 g for 30 min, the
pellet was collected and resuspended in 8 mol/L urea, 0.1 mol/L NaH2PO4, 10 mmol/L Tris-HCl, pH 8.0. Centrifuged again as
before, the supernatant was loaded
on a Ni2+-nitrilotriacetic acid-agarose column (Qiagen). The recombinant protein was eluted from
the column according to manufacturer's instructions. To achieve refolding,
the purified protein was adjusted to pH 8.0 and DTT was added to the
final concentration of 0.01 mol/L.
Following incubation at room temperature for 2 h, the solution was added to refolding
buffer (0.1 mol/L Tris-HCl, pH
8.0, 0.5 mol/L arginine, 5 mmol/L EDTA, 1 mmol/L GSSG, 5 mmol/L GSH) with the ratio of 1∶200[9]. After 24 h incubation at room temperature, the renatured protein was dialyzed
against PB buffer (10 mmol/L Na2HPO4, 10 mmol/L NaH2PO4, pH 7.2) for 24-48
h and lyophilized.
1.5
Bovine aortic endothelial cell proliferation assay
Bovine
aortic endothelial cells were isolated as previously described[8]
and maintained in DMEM supplemented with 10% heat-inactivated FCS and
antibiotics. Monolayer of BAE
cells growing in 60 mm dish was dispersed in 0.05% trypsin solution. Cells were resuspended with DMEM
containing 2% FCS. Approximately 3
000 cells in 200 ml
were added in triplicate to each well of 96-well tissue culture plates and
incubated at 37 ℃
(in 5% CO2). Cells
adhered to the plate in about 2-3
h. The medium was replaced with
200 ml
of fresh DMEM containing 2% FCS, 5
mg/L
bFGF, and samples of recombinant
restin or endostatin were added to each well. After 72 h incubation,
10 ml
MTT (100 g/L) was added to each well and incubated for another 4 h at 37 ℃, 5% CO2. 180 μl
medium was pipetted out from each well and 50 μl
DMSO was added, vortex gently to
dissolve the pellet[9, 10]. The absorbency A570, which correlates to the number of
cells, was measured with
microplate reader (Model 450,
Bio-Rad).
1.6
Cell cycle analysis
All
the procedures were followed as previously reported[11]. Briefly, BAE cells were maintained in DMEM supplemented with 10% FCS
till to 60%-70%
confluence. The medium was changed
with DMEM supplemented with 2% FCS containing 2 mg/L recombinant restin and
bFGF was added to the final concentration of 5 mg/L. After 24 h incubation, cells were trypsinized and washed
gently with PBS, and then were
fixed with 70% ice-cold ethanol for 30 min. Cells were collected by centrifugation. 200 ml
1 g/L RNase was added and incubated at 37 ℃
for 30 min, followed by staining
with propidium iodide at 5 mg/L.
Cells were assessed by FACStar plus flow cytometer (Beckton-Dickinson)
and the results were analyzed with CellQuest software[11].
1.7
Annexin V-FITC binding assay
Annexin
V-FITC (Clontech, Palo Alto, CA) binding assay was performed
according to manufacturer's instruction.
2.5×105
cells were plated onto a 60 mm dish in DMEM containing 2% FCS. After 24 h incubation at 37 ℃, 5% CO2, the medium was changed with DMEM
supplement with 2% FCS, 5 mg/L
bFGF and 2 mg/L recombinant restin.
After 24 h incubation,
cells were trypsinized and were washed in PBS and resuspended in binding
buffer (10 mmol/L HEPES/NaOH, pH
7.4, 140 mmol/L NaCl, 2.5 mmol/L CaCl2). Annexin V-FITC was added to a final
concentration of 100 mg/L, and the cells were incubated in the
dark for 10 min. For each
sample, minimums of 10 000 cells
were counted. Data analysis was
performed with standard Cell Quest software[12, 13].
2.1
Cloning the restin gene in expression plasmids
The
mouse restin gene was amplified by RT-PCR using total RNA from mouse muscle
with the primers we designed. The
cDNA encodes a portion of 184 amino acids corresponding to the amino acid
positions 1 132 to 1 315 of the collagen XV. The amplified cDNA fragment was cloned into the E.coli
expression vector pQE32. DNA
sequence analysis indicated that the desired plasmid had been obtained.
2.2
Purification and characterization of recombinant mouse restin
Recombinant
protein mouse restin plus six histidine was expressed in E.coli and
purified using Ni2+-nitrilotriacetic acid-agarose column and was
refolded in vitro (Fig.1).
Under reducing condition,
recombinant restin migrated in SDS-PAGE with molecular mass of about 22
kD, corresponding to the predicted
molecular mass.
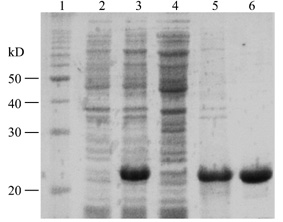
Fig.1 Recombinant
production of mouse restin plus six histidine in E.coli
15% SDS-PAGE Coomassie blue staning; 1, moleculor
weight marker; 2, uninduced cells; 3, induced cells; 4, soluble protein; 5,
insoluble protein; 6, purified protein.
His-restin
and endostatin were assayed for their inhibitory activities on bovine aortic
endothelial cell growth stimulated by bFGF. As shown in Fig.2,
both endostatin and restin inhibited BAE cell proliferation in a
dose-dependent manner, but the
inhibitory activity of restin was weaker than that of endostatin. While both restin and endostatin has no
inhibitory activities on fibroblast cell line Balb/c3T3 and hepatoma cell line
7404 (data not shown), which
suggested that their inhibitory activity was specific to endothelial cell.
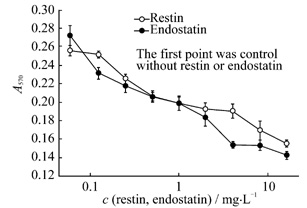
Fig.2 Recombiant mouse restin and endostatin
inhibit the proliferation of BAE cells stimulated by bFGF (n=3)
As
shown in Fig.3, BAE cells were
treated with 2 mg/L restin overnight in 0.5% FCS. The cell cycle assay
demonstrated that 50.9% of cells wasblocked in the G0/G1
after a 24 h treatment with restin, compared with 29.8% of control. The
percentage of S phase cell was 31.2%, compared with 46.8% of control. These
results may explain partly the anti-proliferation effect of restin on BAE cell.
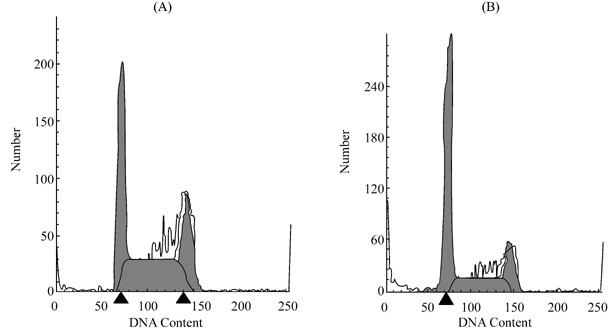
Fig.3 Flow cytomery of BAE cell treatment
with restin
BAE cell monolayers were exposed 24 h to restin
in DMEM supplement with 2% FCS, and cells were assessed by FACStar plus flow
cytometer (Decton-Dickinson). (A) control without restin. (B) cells treated
with restin (2 mg/L).
Annexin
V, a calcium-dependent
phospholipid-binding protein with a high affinity to phosphatidylserine
(PS), was used to detect the early
stage apoptosis[13]. As
shown in Fig.4, treatment with 2
mg/L recombinant restin or endostatin in 2% FCS caused BAE cell apoptosis. At the same concentration, the apoptosis-inducing activity of
endostatin was greater than that of restin, corresponding to the result of proliferation assay.
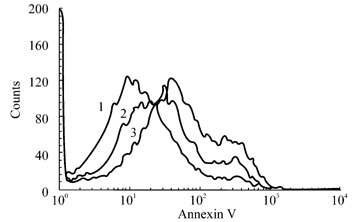
Fig.4 Annexin V-FITC binding assay
BAE cell were treated for 24 h with 2
mg/L restin (2) or 2 mg/L endostatin (3) or an equal volume of PBS(1). Detached
cells and attached cells were collected, and phosphatidylserine externalization
was mesured by labeling with FITC-labeled annexin V. The righword shift on the
x axis of the restin and endostatin peaks indicates increased annexin V-FITC
binding of apoptotic cells.
Angiogenesis
is required for tumors to grow beyond a few millimeters in size[14]. Numerous studies have shown that both
primary tumor growth and metastasis is angiogenesis dependent[1]. Advantages of anti-angiogenic therapy
include ease of access of drugs to the endothelial cell compartment and
lessening the chance of drug resistance.
A number of angiogenesis inhibitor has been identified. Such as angiostatin and endostatin are
fragments of proteins.
Endostatin, a 20 kD
C-terminal fragment of collagen XVIII,
is a specific inhibitor of endothelial cell proliferation and migration.
Restin, a homology protein of endostatin, is the C-terminus of the collagen
XV. Reports by Ramchandran et al[7]
showed that the human restin could inhibited the migration of endothelial cells
in vitro and angiogenesis in mouse model, but didn't inhibit the proliferation of endothelial
cells. In this report, we amplified the gene of 3′terminus
of collagen XV from mouse muscle total RNA. The gene was cloned into pQE32 plasmid. The recombinant protein was expressed, purified and refolded. The recombinant protein inhibited BAE
cell growth as a dose-dependent manner.
Endostatin was reported to induce endothelial cell apoptosis[15]. We here demonstrated that mouse restin
could induce BAE cell apoptosis,
too. Cell cycle analysis of
BAE cells cultivated in medium with mouse restin showed a cell arrest mainly in
the G0/G1 phase.
These results correlate with the BAE cell proliferation assay described
above. There are 20 amino acids
different between mouse and human restin (Fig.5), and these difference may be the reason why the two proteins
have the different biological activity.

Fig.5 The sequence alignment of mouse and
human endostain, restin
The
same amino acids residues are marked with capital letter.
1 O'Reilly MS, Boehm T, Shing
Y, Fukai N, Vasios G, Lane WS, Flynn
E et al. Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell, 1997, 88: 277-285
2 Boehm T, Folkman J,
Browder T, O' Reilly MS. Antiangiogenic therapy of experimental
cancer does not induce acquired drug resistance. Nature,
1997, 390: 404-407
3 Dhanabal M, Ramchandran R,
Volk R, Stilman IE, Lombardo M, Iruela ML,
Simons M et al. Endostatin: Yeast production,
mutants, and antitumor
effect in renal cell carcinoma. Cancer
Res, 1999, 59: 189-197
4 He ZY, Cheng ZY, Qiu
CP, Li B, Zhang WJ, Wu XF,
Cloning, expression and
tumor suppression of human endostatin.
Acta Biochim Biophys Sin,
2000, 32: 333-336
5 Myers JC, Dion AS,
Abranham V, Amenta PS. Type XV collagen exhibits a widespread distribution
in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res, 1996, 286: 493-505
6 Hagg PM, Horelli-Kuitunen N,
Eklund L, Palotie A, Pihlajaniemi T. Cloning of mouse type XV collagen
sequences and mapping of the corresponding gene to 4B1-3. Comparison of mouse and human alpha 1
(XV) collagen sequences indicates divergence in the number of small collagenous
domains. Genomics, 1997, 45: 31-41
7 Ramchandran R, Dhanabal M, Volk R,
Waterman MJ, Segal M, Lu H, Knelbelmann B,
Sukhatme VP. Antiangiogenic
activity of restin, NC10 domain of
human collagen XV: Comparison to
endostatin. Biochem Biophys Res
Commun, 1999, 255: 735-739
8 Pepper MS, Montesano R, el
Aoumari A, Gros D, Orci L, Meda P.
Coupling and connexin 43 expression in microvascular and large vessel
endothelial cells. Am J Physiol, 1992, 262:
C1246-1257
9 Xin L, Xu R, Zhang
Q, Li TP, Gan RB. Kringle 1 of human hepatocyte growth factor inhibits bovine
aortic endothelial cell proliferation stimulated by basic fibroblast growth
factor and causes cell apoptosis. Biochem
Biophys Res Commun, 2000, 277: 186-190
10 Xin L, Zhang L, Xu
R, Zhang Q, Ye Q, Li ZP, Gan
RB. Expression of human
angiostatin in Pichia pastoris and the detection of its
anti-angiogenesis activity. Acta
Biochim Biophys Sin,
2001, 33: 291-295
11
Colorado PC, Torre A, Kamphaus G, Maeshima Y,
Hopfer H, Yakahashi K, Volk R et al.
Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res, 2000, 60: 2520-2526
12 Dhanabal M, Volk R,
Ramchandran R, Simons M, Sukhatme V P. Cloning,
expression and in vitro activity of human endostatin. Biochem Biophys Res Commun, 1999, 258: 345-352
13 Kamphaus GD, Colorado PC,
Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y et
al. Canstatin, a novel matrix-derived inhibitor of
angiogeneisis and tumor growth. J
Biol Chem, 2000, 275: 1209-1215
14 Xin L, Xu R, Gan
RB. Two new angiogenesis
inhibitor. Chemistry of Life,
1999, 19: 21-25
15 Dhanabal M, Ramchandran R, Waterman MJ,
Lu H, Knelbelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell
apoptosis. J Biol Chem, 1999, 274: 11721-11726
16 MacDonald NJ, Shivers WY,
Narum DM, Plum SM, Wingard JM, Fuhrmann SR,
Liang H et al. Endostatin binds tropomyosin. A potential modulator of the antitumor
activity of endostatin. J Biol
Chem, 2001, 276: 25190-25196
Received: September 7, 2001 Accepted: October 22, 2001
*Corresponding author: Tel,
86-21-64374430-5325; Fax,
86-21-64338537; e-mail,
[email protected]