|
http://www.abbs.info e-mail:[email protected] ISSN 0582-9879 ACTA BIOCHIMICA et BIOPHYSICA SINICA 2002, 34(4): 411-417 CN 31-1300/Q |
Cloning
and Characterization of a Novel Human Secretory Protein: Secretogranin III
(
1Research Institute of Biochemistry, State Key Laboratory of
Bioreactor Engineering, East China University of Science
and
Technology, Shanghai 200237, China;
2Chinese National Human Genome Center, Shanghai 201203, China )
SgIII
is the newly found member of the granin family. It was reported originally in rat,
while human SgIII has not been studied[8]. We have found a novel
pituitary protein previously in the study of gene expression profiling in human
tissues of hypothalamus-pituitary-adrenal axis[9]. The protein has
been called human SgIII according to its homology to SgIII of mouse and rat.
Its homologue in rat has been identified which is present in the storage
vesicles of many neuroendocrine cells, especially in the neurons participating
in auditory, olfactory and extrapyramidal motor functions, as well as in
neurons related to the hypothalamic-pituitary axis[8]. The study of Xenopus
laevis SgIII revealed that it is a sulfated protein undergoing proteolytic
processing in the regulated secretory pathway.
In
this paper we further investigate the characterization of human SgIII, a novel
member of granin family. The tagged human SgIII was predominantly localized in
the endoplasmic reticulum and secreted out of the COS-7 cell. As demonstrated
in Northern blot, human SgIII is expressed in heart, skeletal muscle, kidney,
liver and strongly in brain with specific transcripts of higher molecular weight.
1.1
Identification, sequencing, and sequence analysis of human secretogranin
III
Sequencing
was performed using the Applied Biosystems Taq DyeDeoxy Terminator
sequencing kit and ABI 377 automated sequencer. Computer analysis of sequences
was performed with the Wisconsin Package Version 10.0, Genetics Computer Group
(GCG), Madison, Wisc and several shared softwares, such as SignalP v.2.0
(http://www.cbs.dtu.dk/services/SignalP-2.0/)[10,11]. Similarity
searches were performed using the BLAST program.
1.2
cDNA cloning and expression plasmid
The
full-length SgIII coding sequence was amplified by polymerase chain
reaction from the cDNA library of pituitary and hypothalamus (Clontech). PCR
primers (forward, XbaI, 5′GC-TCTAGAAGCCGAGCGTGGAAGAAT3′;
reverse, KpnI, 5′GGGGTACCCAGGCTGCTATAAATGC-GCTT3′)
were used. Amplifications were carried out in a PE9700 thermal cycler with 94 ℃
for 3 min, 35 cycles of 94 ℃
for 30 s, 53 ℃
for 40 s, 72 ℃
for 2 min and a final extension at 72 ℃
for 10 min. For mammalian expression, the fragment was inserted into
pcDNA3.1(-)/Myc-HisA vector (Invitrogen) to generate a plasmid encoding SgIII
with a Myc-His-tag at the C terminus.
1.3
Expression analysis
Human
12-lane multiple tissue Northern (MTN) blot (Clontech), which contain 1mg
poly A+ RNA from one of 12 human tissues per lane, was hybridized
according to manufacturer's protocol. The full length coding sequence of the SgIII
gene generated by PCR amplification, was labeled using Random Primer DNA
Labeling Kit Ver.2 (TaKaRa) as a probe. The blot was washed in 2×SSC/0.5
g·L-1
SDS or 0.1×SSC/1 g·L-1
SDS and exposed to phosphor screen (Molecular Dynamics) for 24 h at room
temperature.
1.4
Immunoblotting
The
culture medium of transfected COS-7 cells were collected while the cells were
washed two times with cold PBS (pH 7.4) and lysed with single detergent lysis
buffer. The culture medium and the cell lysates were purified using TALON metal
affinity resins (Clontech) according to the manufacturer's manual. Proteins
were run on 12% SDS-PAGE and transferred electrophoretically onto PVDF membrane
(Amersham Life Science). Membranes were incubated with monoclonal antibody to
human c-Myc (0.2 g/L, Clontech) following a horseradish peroxidase-linked
secondary antibody and detected using chemiluminescent substrate (ECL,
Amersham).
1.5
Immunocytochemistry.
COS-7
cells were cultured in 10% fetal bovine serum/DMEM in 5% CO2. Plates
seeded in 1.5×105
were grown overnight, and cells were transfected with 2 mg
DNA using lipofectamine (Gibco BRL) according to the manufacturer's protocol.
Transfected cells were fixed with 2% parafor-maldehyde (PFA) /Triton-X-100 and
immunostained with monoclonal antibodies to human c-Myc (0.2 g/L, Clontech)
following the fluorescein-conjugated goat-anti-mouse IgG (0.5 g/L,
Gibco BRL), respectively. The staining patterns were viewed with a Laica
microscope.
2.1
Cloning and characterization of the human secretogranin III gene
Human
SgIII (accession no. AF453583) gene is an open reading frame of 1 404
nucleotides in length, encoding a 468-amino-acid protein (Fig.1), which has
predicted weight of 53 kD and isoelectric point of 4.8. A search of the GenBank
database identified three homologues, Mus musculus SgIII
(accession no.NM_009130), Rattus norvegicus (accession
no.U02983), and Xenopus laevis SgIII (accession no.X92872). The overall
identity between human SgIII with the three homologues are in orderly 87%, 87%,
and 56% at the amino acid level. Alignment of these sequences is depicted in
Fig.2. SgIII showed high identity to other members of granin family in the
region of secretogranin motif (325aa-348aa)
(Fig.3)[8,12]. Sequence analysis of SgIII showed it has three
potential sites (60, 346 and 350 sites) for N-linked oligosaccharides
(Asn-X-Ser/Thr). Moreover, a 19-amino-acid region that highly resembles a
signal sequence exists in N-terminal by analysis of SignalP. The analysis results showed the signal
peptide probability is 89.7% and max cleavage site probability is 0.355 between
position 26 and 27 residues[10,11]. In line with the presence of tyrosine sulfation site in Xenopus
laevis[3], human SgIII also contains a putative tyrosine
sulfation site at residue 123. Other domains and O-linked oligosaccharide
sites are not been found.

Fig.1 Nucleotide and predicted amino acid
sequence of human SgIII
The predicted N-terminal signal sequence
is indicated in italics, and its putative cleavage site is indicated
with an asterisk. Putative poly(A) addition signals are boxed. Tandem
basic residues are indicated by line; the repeated DSTK elements are
indicated by dot line. The potential poly(A) addition sites are marked with
an arrowhead.
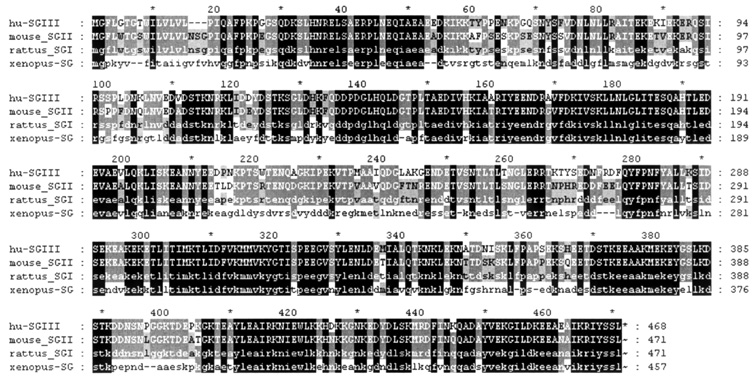
Fig.2 Amino acid sequences comparison of
human SgIII with other homologs
Identical
residues are in black, and conserved substitutions are in gray.

Fig.3 Comparison with secretogranin motif
The regions of secretogranin motif are
compared here: hSgIII (residues 325-348),
hCgA (human chromogranin A residues 406-427),
hSgI (human secretogranin I residues 650-672),
hSgII (human secretogranin II residues 488-512).
Gaps have been introduced to optimize the alignments.
By
searching the human expressed sequence tags (EST) database and comparison with
human genome database, we found three potential cDNA forms resulting from
possible different poly (A) addition sites in the last exons in the 3′
untranslated region (UTR). The two short forms, terminated in the positions 1
908 bp and 2 017 bp respectively, were coincident with the two SgIII
transcripts 2.2 kb and 1.9 kb in rat. The long form cDNA, 3 371 bp in
length, was supported by ESTs most from brain.
Since
it has been reported that SgIII is concentrated in brain, especially in
pituitary, we obtained the gene from pituitary and hypothalamus cDNA library
(Clontech) by PCR, then cloned it into pcDNA3.1A.
2.2
Genomic organization of the human secreto-granin III gene
Comparisons
of the human SgIII cDNA sequence with GenBank genome database identified
a genomic clone NT010204.6 derived from human chromosome 15. The human SgIII
locates in chromosome 15q21.3 and has 12 exons and 11 introns. As demonstrated
in Fig.4, the 12 exons from the known genomic SgIII clone are spread over more
than 39 kb pairs, with the largest intron at approximate 12 kb between the 10th
exon and the 11th exon.
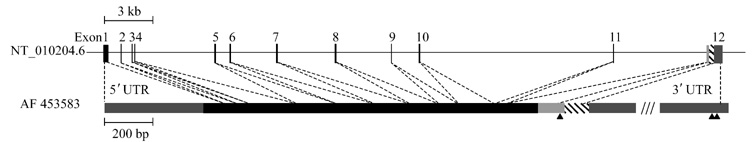
Fig.4 Intron-exon structure of the SgIII gene
The genomic SgIII clone is derived
from chromosome 15. (accession no. NT_010204.6). The coding exons of human SgIII
are depicted as black proportional bars, with gray portions representing
noncoding regions and black portion representing coding region. The last exon
contains three possible poly(A) addition sites; they are indicated with
different patterns. The putative poly(A) addition signals are indicated with an
arrowhead. The slash marks indicate the last exon of the longest form of SgIII,
which is approximately 1.6 kb.
2.3
Expression analysis
The
tissue expression of human SgIII mRNA was examined by Multiple Tissue
Northern blot using the full-length SgIII cDNA as a probe. The SgIII
was detected strong hybridization to a 2.2 kb band and weaker hybridization to
a 1.9 kb target (Fig.5). But in samples from the brain, it is of interest that
four mRNA size variants are present, we detected specific hybridization to 4.5
kb and 3.3 kb bands, while 2.2 kb and 1.9 kb bands were very weak. There are
similar levels of mRNA expression in small intestine, placenta, colon and
apparently higher levels in brain, heart, skeletal muscle, kidney and liver. It
has been reported rat SgIII gene gives rise to 2 stable mRNAs 2.2
kb and 1.9 kb,which differ only in their sites of 3′
polyadenylation[8]. Accordingly, we think the 2.2 kb and 1.9 kb
mRNAs of human SgIII were possible due to the difference of poly (A) addition
sites. The 3.3 kb transcript in brain agreed with the long cDNA form which was
assembled by ESTs mainly from the brain.
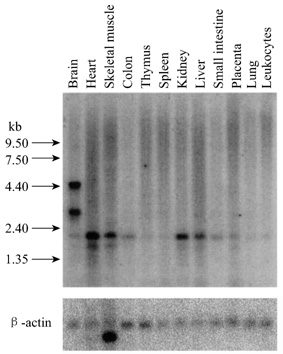
Fig.5 Distribution of SgIII mRNA in human
tissues
RNA blot containing poly(A+)
RNA from multiple human tissues was hybridized with 32P-labled human
SgIII cDNA as a probe. Human b-actin
was used as a control to determine the relative amount of RNA from each tissue.
2.4
Cellular localization of secretogranin III protein
COS-7
cells were transiently transfected with the pcDNA-SgIII plasmid to examine the
cellular localization of the c-Myc-tagged SgIII protein. The localization of
expressed protein was visualized using the antibody c-Myc 9E10 and secondary
antibody goat anti-mouse IgG (FITC labeled). Although a part of synthesized
SgIII was secreted into medium, most of expressed proteins remained
intracellular. The tagged SgIII was localized mainly in the perinuclear region
as well as a prominent tubular network (Fig. 6), a pattern characteristic which
is identical with ER[13,14], and no fusion protein was detected in
nucleus.
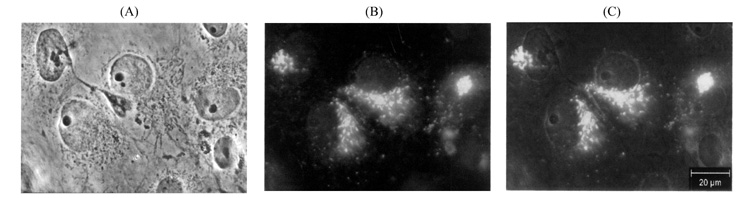
Fig.6 Immunofluorescence detect of SgIII
expressed in COS-7 cells
COS-7 cells, which were transfected with
pcDNA-SgIII, were stained with anti-c-Myc antibody and visualized with a fluorescein
isothiocyanate-conjugated antibody (B). (A) is a common view of the stained
cells. Staining profiles were oberved using a Laica microscope. (C) is an
overlap of (A) and (B).
SgIII
contains a possible cleavable signal peptide, and no hydrophobic transmembrane
segment, which is consistent with transfer of the protein into the lumen of the
ER. To verify that the N-terminal stretch of hydrophobic amino acids function
as a signal sequence, we tested the expression of SgIII by immunoblotting with
human growth hormone as a positive control (Fig. 7). Both in cell lysates and
in medium supernatants the tagged SgIII could be detected. Non-specific bands
were detected in the lanes of intracelluar pcDNA3.1(-)MycHisA and HuGH-Myc-His.
The fusion protein in the extracellular fluid migrated at approximately 63 kD
that is greater than the intracellular protein. Previously, the data base
search showed SgIII has three potential N-glycolysation sites, so this
result indicates SgIII may has been glycosylated during the process of
secretion.
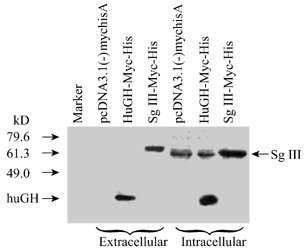
Fig.7 Immunoblotting analysis of the tag
SgIII expressed in COS-7 cells
The expression of the tagged SgIII in
COS-7 cell was examined by immunoblotting with human growth hormone
(HuGH-Myc-His) as a positive control and pcDNA3.1(-)MycHisA as a negative
control.
3
Discussion
In
this study we have characterized human SgIII gene, encoding a 468-amino
acid protein with an N-terminal signal sequence. The abundance of acidic
residues in the polypeptide leads to an acidic isoelectric point that may
contribute to the SgIII taking effect in the low pH environment of trans-Golgi
network (TGN). It is known that other established granins, CgA, SgI and SgII
also have many acidic residues and low pH can promote the calcium-induced
aggregation of human SgII[14,15]. The negative charges of human
SgIII may favor its function in the acidic physiological lumenal milieu of
secretory granules. Additionally, human SgIII has seven pair dibasic sites that
are potential cleavage sites in the posttranslational modifications by
endoproteolytic enzymes[2]. Sequence alignment with the SgIII
homologues indicates that human SgIII is conserved at DSTK repeated sequences,
that is reminiscent of a repeat present in the trans-Golgi network integral
membrane proteins TGN38 and TGN41, a finding more consistent with an
intracellular function for this protein[3]. Moreover, SgIII contains
the obvious secretogranin motif (Fig.4). The biological significance of this
motif remains to be investigated. Subcelluar localization and immunoblotting
indicates SgIII is secreted into the
cell supernatants through TGN. This result matches the function that
granins participate in the packaging and sorting of some neuropeptides and the
formation of secretory granules in the TGN[4,17].
Although
human SgIII shares many structure features with other homologues and members of
granin family, the dibasic sites of human SgIII are not consistent with those
of other homologues, such as Xenopus laevis SgIII[3].
Additionally, its tissue distribution is more widely and two specific 4.5 kb
and 3.3 kb transcripts only exist in brain. In contrast, rat SgIII is
expressed specifically in brain with two variants of 2.2 kb and 1.9 kb[8].
We have found three cDNA forms of human SgIII by EST analysis. Poly(A)
tail could appear behind the sites of 1 908, 2 017 and 3 314 bp of this gene
respectively, that will generated at least three transcriptional products
represented 1.9 kb, 2.2 kb and 3.3 kb hybridization bands in Northern blot. The
number of ESTs represented the 2.2
kb transcriptional product was much more than that of 1.9 kb one. So the 2.2 kb
cDNA was predominant and might play primary role in most of human tissues
examined while the 1.9 kb transcript might be appurtenant (coincidentally, the
ESTs related to the 2.0 kb cDNA form are predominant in most of human tissues).
And most of ESTs related to the 3.3 kb form are just from brain. So it can
illustrate the result of strong and specific hybridization to 3.3 kb band only
in brain. We cannot find other cDNA form correlated to 4.5 kb band. But we
still can predict that this 4.5 kb transcript, as the 3.3kb one, is possible
due to the different transcriptional termination in the last exon or a special
splicing pattern of pre-mRNA. Despite the variation in the 3'UTR, the CDS of
human SgIII gene keep constant. Since splicing of pre-messenger RNA is
regulated differently in the brain compared with other tissues and it is
significant in many cases[18], further work will be required to find
the role of brain specific transcripts.
Genetic
ablation of the SgIII gene in mice had not obvious effects on viability,
fertility or locomotor behavior[19,20]. Many of the cell types that
normally express SgIII can survive and function without this protein, perhaps
because the normal functions of SgIII can be replaced by the products of other
genes. Joost et al. have reported the mRNA levels of Xenopus laevis
SgIII in intermediate pituitary increased in parallel with that of
proopiomelanocortin when changing the background color of the toad[3,21].
This finding shows SgIII may have a role in the production and release of
peptide hormones. Moreover, the endoproteolytic processing of SgIII is a
widespread phenomenon in the neuroendocrine system of vertebrates. SgIII may be
trigger complex dissociation in its processing and facilitating maturation of
the granular contents as a helper protein. Since the function of SgIII is still
elusive, further investigation is needed to determine the role of SgIII in the
regulated secretory pathway.
Acknowledgements We acknowledge Dr. Zhang X
for her help with our work. We also thank Dr.Xu WQ at Institute of
Neuroscience, Chinese Academy of Sciences for photographing.
1 Huttner WB, Gerdes HH, Rosa P. The
granin (chromogranin/secretogranin) family. Trends Biochem Sci, 1991, 16(1):
27-30
2 Muller L, Barret A, Picart R, Tougard
C. Proteolytic processing of sulfated secretogranin II in the trans-Golgi
network of GH3B6 prolactin cells. J Biol Chem, 1997, 272(6): 3669-3673
3 Holthuis JC, Jansen EJ, Martens GJ.
Secretogranin III is a sulfated protein undergoing proteolytic processing in
the regulated secretory pathway. J Biol Chem, 1996, 271(30):17755-17760
4 Ozawa H, Takata K. The granin family--its role in sorting and
secretory granule formation. Cell Struct Funct, 1995, 20(6): 415-420
5 Winkler H, Fischer-Colbrie R. The
chromogranins A and B: The first 25 years and future perspectives. Neuroscience,
1992, 49(3): 497-528
6 Arvan P, Castle D. Sorting and storage
during secretory granule biogenesis : Looking backward and looking forward. Biochem
J, 1998, 332: 593-610
7 Dannies PS. Protein hormone storage in
secretory granules: Mechanisms for concentration and sorting. Endocr Rev,
1999, 20(1): 3-21
8 Ottiger HP, Battenberg EF, Tsou AP,
Bloom FE, Sutcliffe JG. 1B1075: A brain- and pituitary-specific mRNA that
encodes a novel chromogranin/secretogranin-like component of intracellular
vesicles. J Neurosci, 1990, 10(9): 3135-3147
9 Hu RM, Han ZG, Song HD, Peng YD, Huang
QH, Ren SX, Gu YJ et al. Gene expression profiling in the human
hypothalamus-pituitary-adrenal axis and full-length cDNA cloning. Proc Natl
Acad Sci USA, 2000, 97: 9543-9548
10 Nielsen H, Engelbrecht J, Brunak S, von
Heijne G. Identification of prokaryotic and eukaryotic signal peptides and
prediction of their cleavage sites. Protein Eng, 1997, 10:
1-6
11 Nielsen H, Krogh A. Prediction of
signal peptides and signal anchors by a hidden Markov model. Proc Int Conf
Intell Syst Mol Biol. 1998, 6: 122-130
12 Gerdes HH, Rosa P, Phillips E, Baeuerle
PA, Frank R, Argos P, Huttner WB. The primary structure of human secretogranin
II, a widespread tyrosine-sulfated secretory granule protein that exhibits low
pH- and Calcium-induced aggregation. J Biol Chem, 1989, 264(20):
12009-12015
13 Ozawa M, Muramatsu T. Reticulocalbin, a
novel endoplasmic reticulum resident Ca(2+)-binding protein with multiple
EF-hand motifs and a carboxyl-terminal HDEL sequence. J Biol Chem, 1993,
268(1): 699-705
14 Munro S, Pelham HR. A C-terminal signal
prevents secretion of lumenal ER proteins. Cell, 1987, 48(5): 899-907
15 Yoo SH. pH- and Ca(2+)-dependent
aggregation property of secretory vesicle matrix proteins and the potential
role of chromogranins A and B in secretory vesicle biogenesis. J Biol Chem,
1996, 271(3): 1558-1565
16 Holthuis JC, Martens GJ. The
neuroendocrine proteins secretogranin II and III are regionally conserved and
coordinately expressed with proopiomelanocortin in Xenopus intermediate
pituitary. J Neurochem, 1996, 66 (6): 2248-2256
17 Calegari F, Coco S, Taverna E, Bassetti
M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in
cultured hippocampal astrocytes. J Biol Chem, 1999, 274(32):
22539-22547
18 Dredge BK, Polydorides AD, Darnell RB.
The splice of life: alternative splicing and neurological disease. Nat Rev
Neurosci, 2001, 2(1): 43-50
19 Kingsley DM, Rinchik EM, Russell LB,
Ottiger HP, Sutcliffe JG, Copeland NG, Jenkins NA. Genetic ablation of a mouse
gene expressed specifically in brain. EMBO J, 1990, 9(2):
395-399
20 Dopazo A, Lovenberg TW, Danielson PE,
Ottiger HP, Sutcliffe JG. Primary structure of mouse secretogranin III and its absence
from deficient mice. J Mol Neurosci, 1993, 4(4): 225-233
21 Martens GJ, Civelli O, Herbert E.
Nucleotide sequence of cloned cDNA for pro-opiomelanocortin in the amphibian Xenopus
laevis. J Biol Chem, 1985, 260(25): 13685-13689
Received:
January 23, 2002
Accepted: April 3, 2002
This
work was supported by the grant from State 863 High Technology R&D Project
of China(No.102-08-08-01)
*Corresponding
authors: HAN Ze-Guang: Tel, 86-21-50801325; Fax, 86-21-50800402; e-mail,
[email protected]; WEI Dong-Zhi: Tel, 86-21-64250068; Fax, 86-21-64250068;
e-mail, [email protected]